Vaccine Science: COVID-19

As the government continues to roll out its COVID-19 vaccination program, it is understandable that you may have questions about the quality, safety and efficacy of COVID-19 vaccines. The objective of this article is to provide you with the science behind the COVID-19 vaccines and the process of gaining their market approval.
The information has been organized under commonly asked questions to help you navigate the information.
This article does not substitute for professional medical advice. Always seek the guidance of your doctor or other qualified health professional with any questions about COVID-19 vaccines.
What is coronavirus?
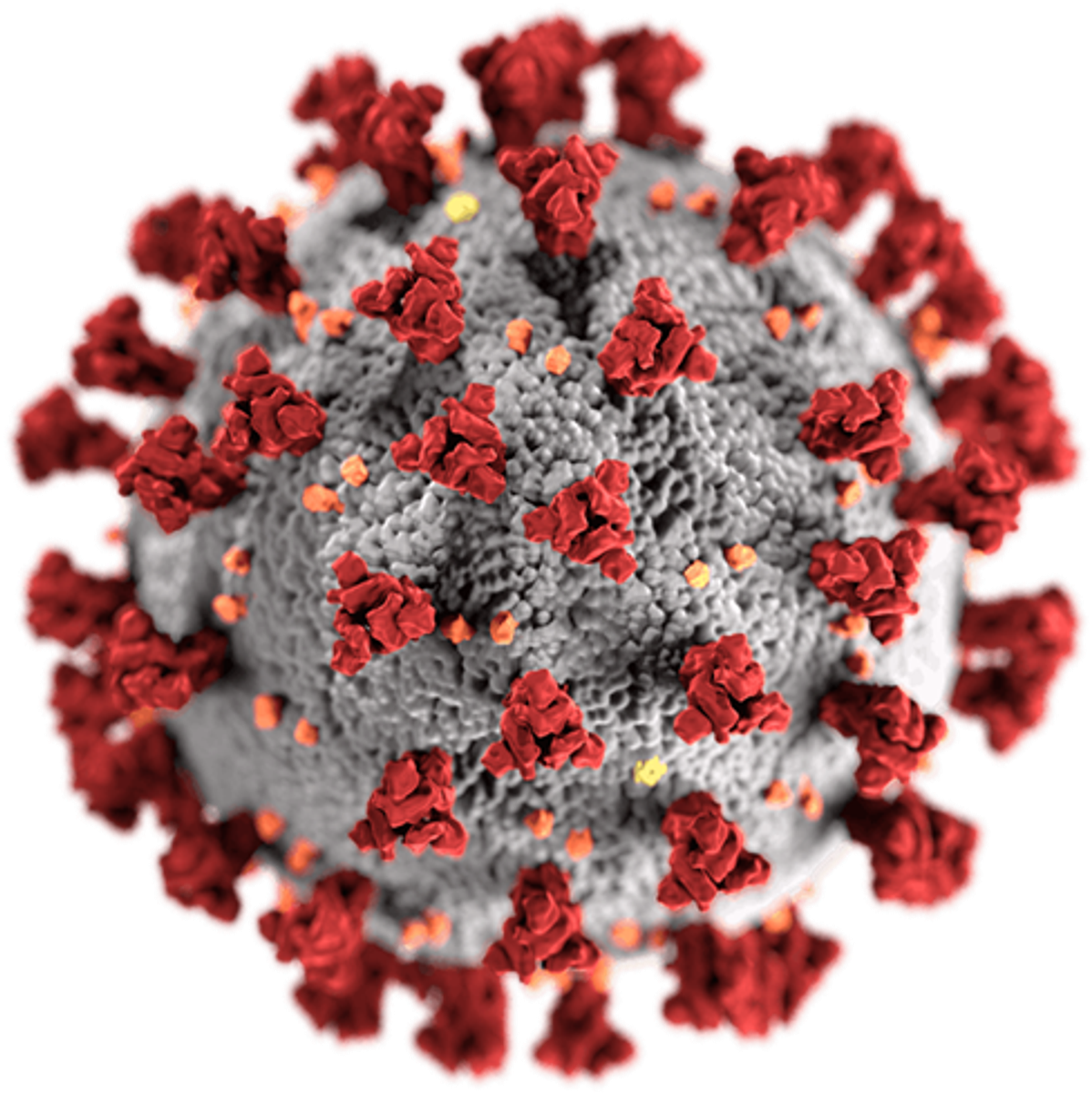
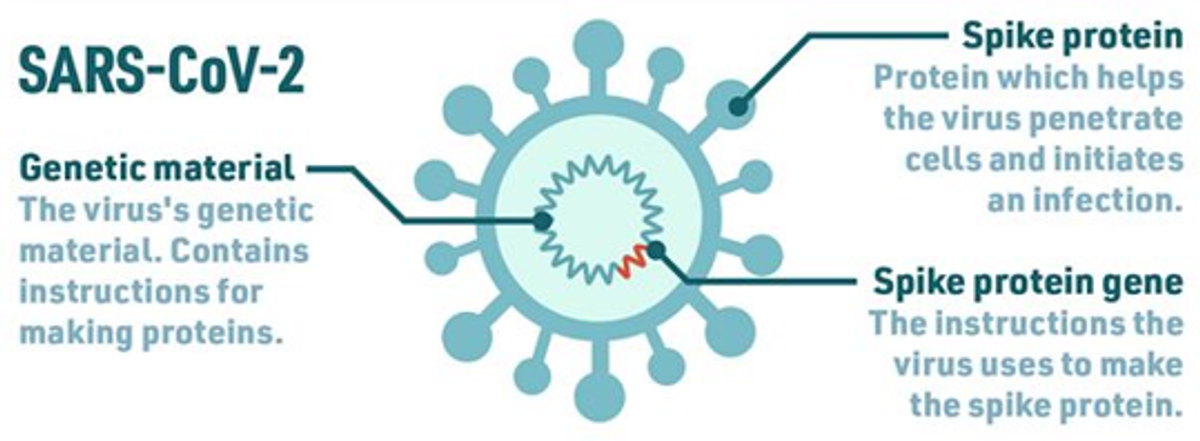
Coronaviruses are a family of viruses that have crown-like ‘thorns’ on their surface. There are many types of coronavirus, but in 2019, scientists identified a novel coronavirus: severe acute respiratory syndrome, or SARS-CoV-2 for short. COVID-19 is the name of the disease that SARS-CoV-2 causes.
Using cryo-electron tomography, scientists have constructed an image of the SARS-COV-2 virus.
The red ‘thorns’ are called spike proteins and the virus uses these to invade the cells in our body. Once inside our cells, the virus reveals its genetic code which tells our cells how to make copies of the virus – which our cells then do.
What scientists have discovered is that the spike protein on a SARS-CoV-2 virus has unique properties (ie. a flexible stalk) which allow it to bind to human cells more strongly and replicate more abundantly in the upper respiratory tract, making it highly transmissible.
How do vaccines treat COVID-19?
All vaccines work by exposing the body to molecules from the target virus to trigger an immune response. COVID-19 vaccines aim to produce immunity to the SARS-CoV-2 virus.
Traditional vaccines, like the measles and seasonal influenza vaccines, inject weakened or inactivated forms of the virus into our bodies. These types of vaccines have well-established technology however, weakened vaccines are unsuitable for people with compromised immune systems. Another drawback of traditional vaccines is that they take a long time to develop, as the viruses need to be grown in the laboratory and treated to ensure they are safe to inject.
Two COVID-19 vaccines approved for use in Australia are: the one developed by Pfizer and the one developed by the University of Oxford in collaboration with AstraZeneca.
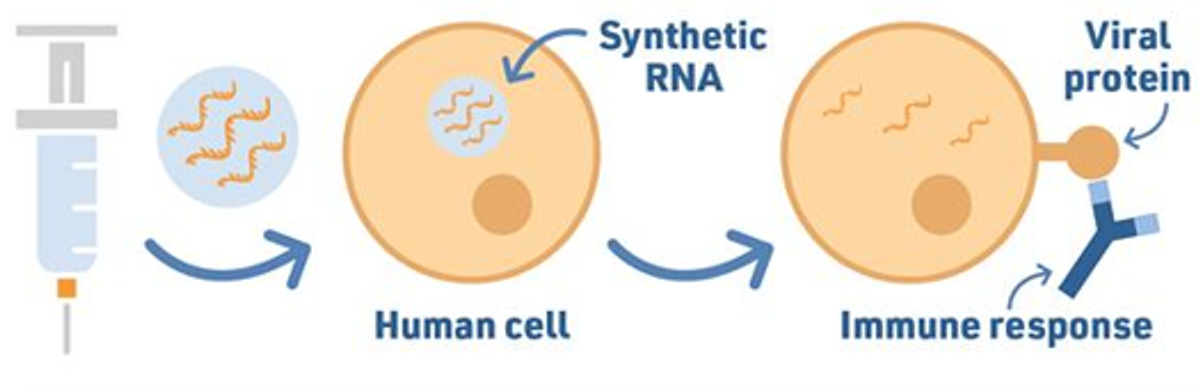
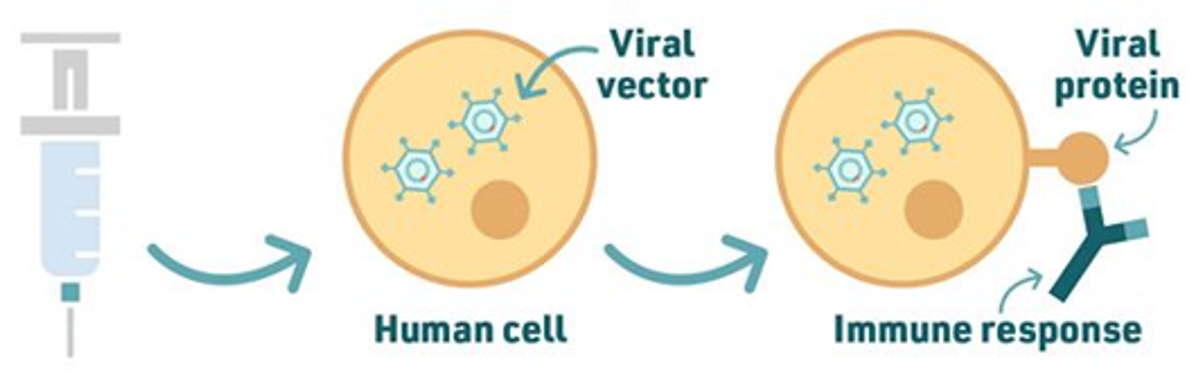
The Pfizer vaccine is an mRNA vaccine. The AstraZeneca vaccine is a viral vector vaccine. Both vaccines operate differently from traditional vaccines as they do not introduce virus (SARS-CoV-2) into the body.
Instead, scientists have analysed the genetic material inside the SARS-CoV-2 virus and have identified and isolated the gene that tells the virus how to make its spike protein.
source: Royal Society of Chemistry
The Pfizer vaccine packages the mRNA of the spike protein gene in lipid nanoparticles (very small fat droplets) so that the cells of our body absorb it without breaking it down first. Once inside, the lipid capsule dissolves and our cell reads the mRNA instructions to make spike proteins on the surface of our cell. This triggers an immune response and our body learns how to destroy spike proteins.
If in future you become infected with SARS-CoV-2, your body will know how to attack the spike protein thus making the virus less effective in replicating in our body.
The drawback with mRNA vaccines is that they have to be stored at very low temperatures (-70°C) to remain stable, which makes storage and transport more challenging.
The AstraZeneca vaccine works in a similar way only instead of using the mRNA of the spike protein, it uses the DNA of the spike protein and adds this to another virus (a viral vector). In the case of the AstraZeneca vaccine, the viral vector - or carrier virus - is a weakened and modified version of a common cold virus (adenovirus) from chimpanzees – so it cannot replicate and cause illness.
Viral vector vaccines are more stable than mRNA vaccines and can therefore be stored in the fridge, making it easier for transport.
The genetic material of the spike protein found in both types of vaccines cannot cause infection because our body quickly breaks it down after reading its genetic code. Also it cannot in any way affect our cell’s DNA because the DNA-to-RNA biological process is one-way.
How did scientists develop a COVID-19 vaccine so quickly?
The biggest misconception about COVID-19 vaccines is that the work started when the pandemic began. The development of mRNA and viral vector vaccines has been building over decades.
In 2005, however, scientists had a breakthrough on how to make mRNA vaccines stable and have since developed mRNA vaccines to treat HIV and some cancers. Known as the plug and play method, scientists could swap out the gene sequence of the HIV antigen and 'plug in' the gene sequence of the SARS-CoV-2 spike proteins.
Finding the right protein is the challenge but the method is always the same.
Prof. Medicine, University of Pennsylvania
This is why mRNA vaccines can be rapidly developed compared with traditional vaccines.
It sounds almost perverse to say, but it was lucky that the pandemic was caused by a coronavirus. This family of viruses have tried to jump from animals to people twice before in the past 20 years – the original SARS coronavirus in 2002 and MERS coronavirus in 2012. Scientists therefore had a head start in understanding the biology of coronaviruses and their spike protein.
But it was the world’s biggest Ebola outbreak in 2014-2016, where the response was too slow and many people died, that health authorities wanted to do better. In the recriminations that followed, a plan emerged on how to tackle the next ‘big one’, or Disease X.
Having already developed a MERS viral vector vaccine, Oxford scientists applied the ‘plug and play’ method to substitute in the genetic code for the spike protein found on the SARS-CoV-2 virus. The Oxford team were not starting from scratch.
If this had been a completely unknown virus, then we'd have been in a very different position.
Prof. Andrew Pollard, Oxford University
How did the COVID-19 vaccines get approved so quickly?
All medicines go through three phases of human clinical trials prior to marketing the product. At each stage, the safety, quality and efficacy is tested. For most medicines, clinical trials can take years due to the involved process of writing applications, gaining approval, negotiating with manufacturers and trying to recruit enough people to take part in the trials. It can take years to get from one phase to the next.
The [clinical trial] process is long, not because it needs to be and not because it's safe, but because of the real world.
Dr Mark Toshner, Cambridge University
As the pandemic tightened its grip on the world and country after country descended into lockdown, money and human resources started to flow. With large sums of money given to vaccine firms by public funders and private philanthropists, they could do all three phases of clinical trials in parallel instead of sequentially.
Furthermore, the regulators, who would normally wait until after the trials were concluded, have been involved in the process early, conducting ‘rolling reviews’ of the safety, manufacturing standards and effectiveness of the vaccines. The COVID-19 vaccines have been through every phase of the clinical trial process that would normally take place for a medicine.
Why do the health authorities keep changing their advice?
The fourth phase of clinical trials occur after the vaccine has been marketed. Phase IV studies are designed to monitor the effectiveness of the approved medicine in the general population and to collect information about any adverse effects associated with widespread use over longer periods of time.
With most countries now rolling out their COVID-19 vaccination program, phase IV data that would normally take years to accumulate for a typical medicine, is accumulating in months for COVID-19 vaccines.
Equipped with more data, the Australian regulators estimated that rare blood clots affect roughly five people in every 1 million of AstraZeneca vaccine recipients, mostly women under 55 years old. Roughly one in four people who developed a clot later died. These rare clots were not picked up in the first three phases of the clinical trials because of sample size.
There will be things that get picked up when you start testing millions of people, rather than thousands.
Prof Magdalena Plebanski, RMIT University
Therefore in April 2021, the Australian regulators recommended that the Pfizer vaccine is preferred over the AstraZeneca vaccine for people under 50 years. This risk assessment was based on the low exposure risk of SARS-CoV-2 in Australia at the time.
With the current outbreak of SARS-CoV-2 in NSW and Victoria and the increased risk of infection, the Australian health authorities reaffirms that the benefits of the AstraZeneca vaccines are greater than the risk of rare side effects for all age groups.
If we were to have a COVID outbreak, then the clotting risks are actually far, far higher in patients that contract COVID itself.
Dr Vivien Chen, University of Sydney
Furthermore, the Australian health authorities currently recommend that individuals strongly consider vaccination at this time due to the Delta variant being more severe than the original SARS-CoV-2 strain. The proportion of people less than 60 years requiring hospitalisation appears to be higher than was reported in outbreaks with the original SARS-CoV-2 strain.
REFERENCES