GS1 Healthcare
Update from an ISQua Member

GS1 Healthcare
Update from an ISQua Member
2020 was a year like no other—one filled with complex challenges and opportunities. GS1’s vision—the use of global standards to help enable optimal quality and efficiency of healthcare delivery to benefit patients—took on a renewed sense of urgency and meaning as researchers, academia and healthcare providers collaborated to advance COVID-19 vaccines and treatments.
Now in 2021, the world is moving forward as multiple vaccines roll out to meet massive and immediate demand. Healthcare providers, government leaders, regulators and public health authorities must come together to ensure the logistics requirements are in place across the global healthcare supply chain. In its white paper, Securing trust in the global COVID-19 supply chain, Deloitte advises, “Trust will be a cornerstone for the successful launch, distribution and acceptance of vaccines.”
Embracing the use of GS1 standards enables trust at all levels of the global healthcare supply chain.
Today, more than 70 countries have traceability systems and healthcare regulations or trading partner requirements to use GS1 standards and identifiers such as GS1 DataMatrix to encode pharmaceuticals, medical devices and personal protective equipment information to reduce errors, and improve efficiency and safety.[1]
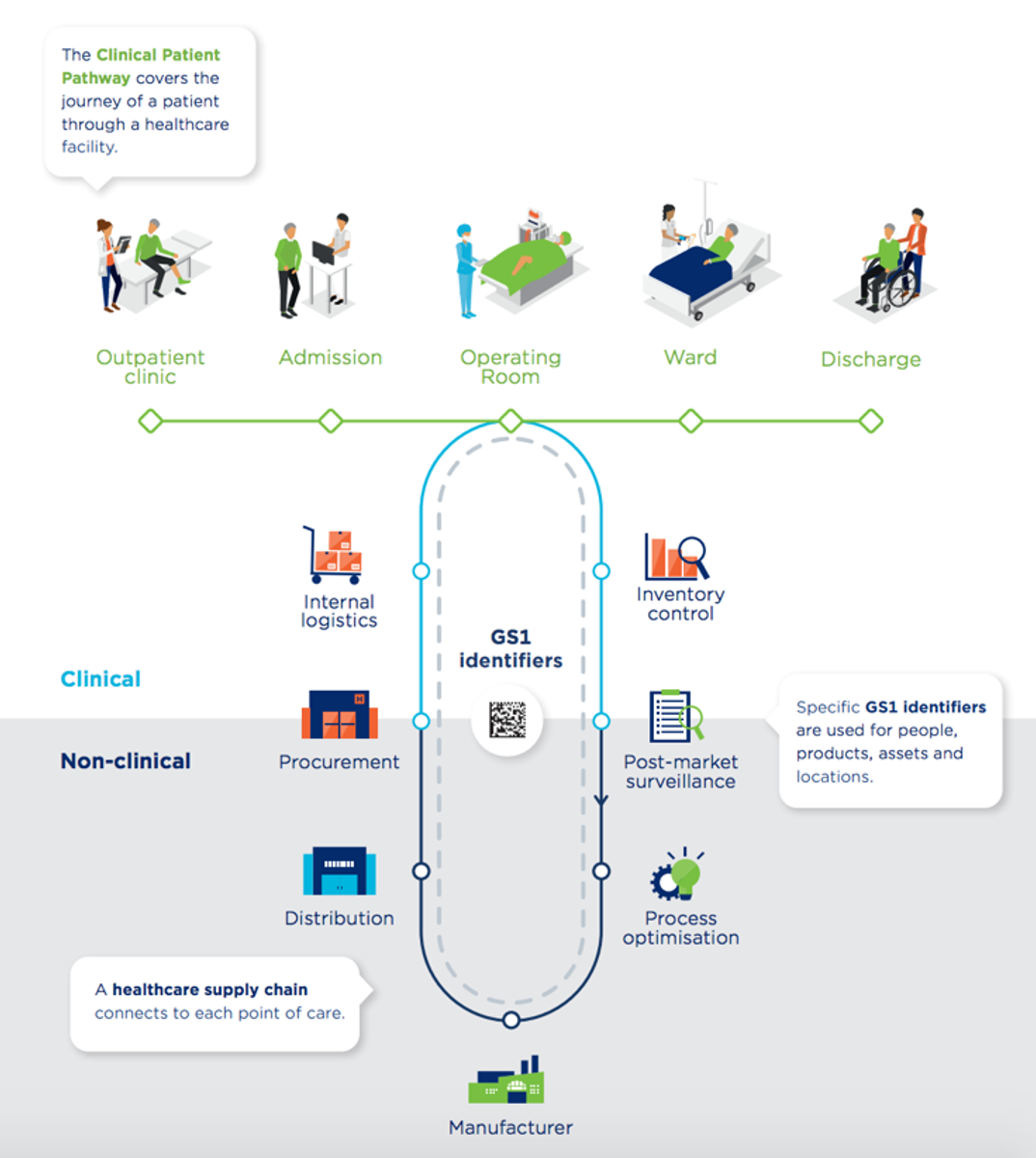

GS1’s Clinical Patient Pathway illustrates the patient’s journey through a hospital and how GS1 standards enable traceability, connecting the supply chain with the hospital’s clinical and business processes. Throughout the journey, GS1 barcodes are scanned to collect trusted information that can be used for improving the patient’s quality of care and safety, and promoting better outcomes.
Trust in the healthcare supply chain enabled by GS1 standards extends to hospital environments.
Hospital Israelita Albert Einstein (HIAE) in Brazil created a traceability system where all individual doses of medicines are assigned a GS1 identifier, batch/lot number and expiration date, encoded in a DataMatrix barcode. Staff and nurses can scan medicines’ barcodes as they travel from receiving to the hospital’s points of care. Since suppliers apply barcodes at their sites, significant, tangible savings have been achieved in both time and costs.[2]
Franciscan Missionaries of Our Lady Health System (FMOLHS) in the US decided to implement GS1 standards to help drive the digital transformation of its critical processes. Today, approximately 85% of implants that come into its warehouse are uniquely identified with GS1 identifiers by suppliers. By scanning an implant’s barcode, information is captured in a patient’s record and outcomes can be matched to products and procedures used. Surgeons can now see what it costs for a procedure, the anticipated patient outcome, and the length of recovery time.[3]

GS1 standards provide the foundation of safe and trustworthy healthcare systems.
The World Health Organization estimates that adverse drug reactions are the fourth to sixth-largest cause for mortality in some countries.[4]
In response to this worldwide challenge of patient safety, the HIMSS Clinically Integrated Supply Outcomes Model (CISOM) offers healthcare providers a prescriptive path forward to restructure clinical environments and promote the transparency of data to proactively identify risks to quality and safety. The model calls for GS1 standards, leadership and policies. In turn, clinicians can take preventive measures to reduce risk and boost the quality of care that may ultimately eliminate medical errors.
Join the discussion!
In 2021, GS1 will continue its work with all healthcare stakeholders to help drive the implementation of global standards. In short, we remain strong in our commitment to the safety and health of patients.
Be part of GS1 Healthcare online events where you can learn from and dialogue with other healthcare professionals about GS1 standards’ impact on quality of care and patient safety.
For more information about GS1 standards, visit the GS1 Healthcare website.
[1] Reh, Greg and Hanno Ronte. (2020). Securing trust in the global COVID-19 supply chain. Retrieved from https://www2.deloitte.com/global/en/pages/life-sciences-and-healthcare/covid-19/securing-trust-in-the-global-covid-19-supply-chain.html.
[2] Malta, Nilson Gonçalves. (2019). Hospital Israelita Albert Einstein continues its journey to full traceability of pharmaceuticals. Retrieved from https://www.gs1.org/sites/default/files/docs/healthcare/Reference-Book/190156_GS1_RB2018_Final_SinglePages_Web_092018.pdf.
[3] Michel, Sandi. (2020). Fulfiling a vision of digital transformation with GS1 standards. Retrieved from https://www.gs1.org/docs/healthcare/Reference-Book/Reference-Book-2020-2021-FINAL-planche.pdf.
[4] World Health Organization. Aide Memoire: For a national strategy for safe drugs and their appropriate use. https://www.who-umc.org/media/1211/aide-memoire-en.pdf