Secondary Science

Year 12 Chemistry
In a recent series of hands-on chemistry practical activities, students explored a range of core concepts related to chemical equilibrium and reaction behavior. They distinguished between the rate and extent of reactions, and observed the dynamic nature of homogeneous equilibria in aqueous and gaseous systems, using balanced chemical and thermochemical equations. Applying Le Chatelier’s Principle, students predicted and demonstrated how equilibrium shifts in response to changes in temperature, concentration, and pressure. Importantly, these practicals will be supplemented with lessons on balancing maximizing yield and optimizing reaction conditions, tying in green chemistry principles such as the use of catalysts and energy-efficient processes. These practicals deepened students' understanding of real-world chemical systems and sustainable chemical design.
Dr Phil Bergen
Chemistry Teacher
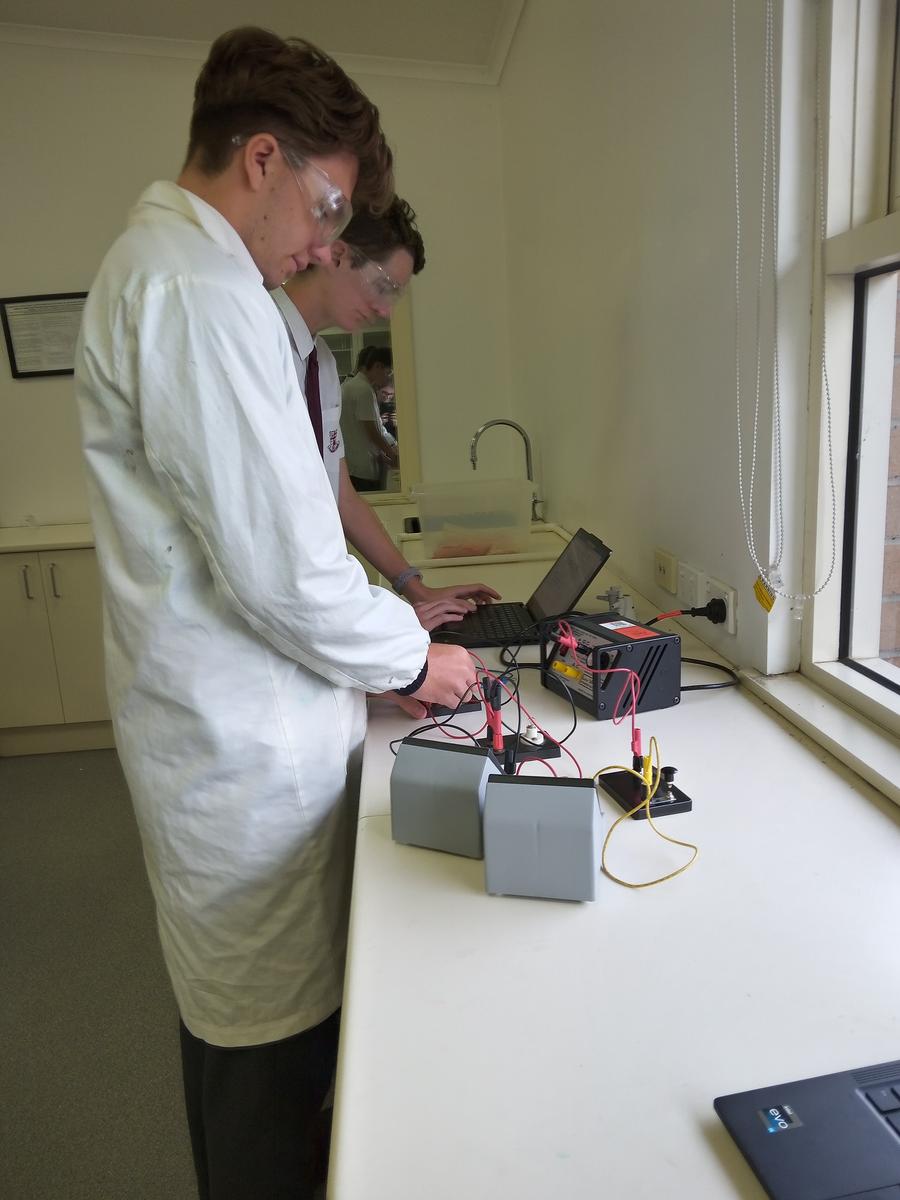
Year 11 Physics
Students in Year 11 Physics have been developing conceptual models to analyse electrical phenomena and its application in household circuits. Recently, students completed an experiment investigating the difference between series and parallel circuits. Students measured the voltage and the current across one of the lightbulbs, then calculated the resistance and estimated the sources of error within the experiment.
Lisa Thomson
Physics Teacher