SCIENCE

SCIENCE TALENT SEARCH 2025
The Science faculty are inviting students to participate in the Science Talent Search (STS). STS is an annual competition that aims to challenge and reward students with an interest in Science. In previous years McKinnon students have had enormous success, winning bursaries up to $100 each.
This year’s theme:
‘DECODING THE UNIVERSE: Exploring the Unknown with Nature’s Hidden Language’
This is a suggestion for a project. While students are encouraged to use it, it is not a requirement (except for the Creative Writing and Poster categories).
Students can enter individually or in pairs. There is a small cost associated with each entry.
Categories are:
Computer Programming/Games and Simulations
Photography
Creative Writing
Videos
Inventions
Experimental Research
Working Models
Posters/Scientific wall charts
See link below for more categories and information. (To access the link you will need a McKinnon email.)
Science Talent Search Category Basic Information
Email Ms Sweeney swt@mckinnonsc.vic.edu.au or Dr Fleming flb@mckinnonsc.vic.edu.au for more information.
To Register your interest fill inthis form by Friday 2 May. (Again, to access the link you will need a McKinnon email).
USING BLUETOOTH SENSORS IN CHEMISTRY
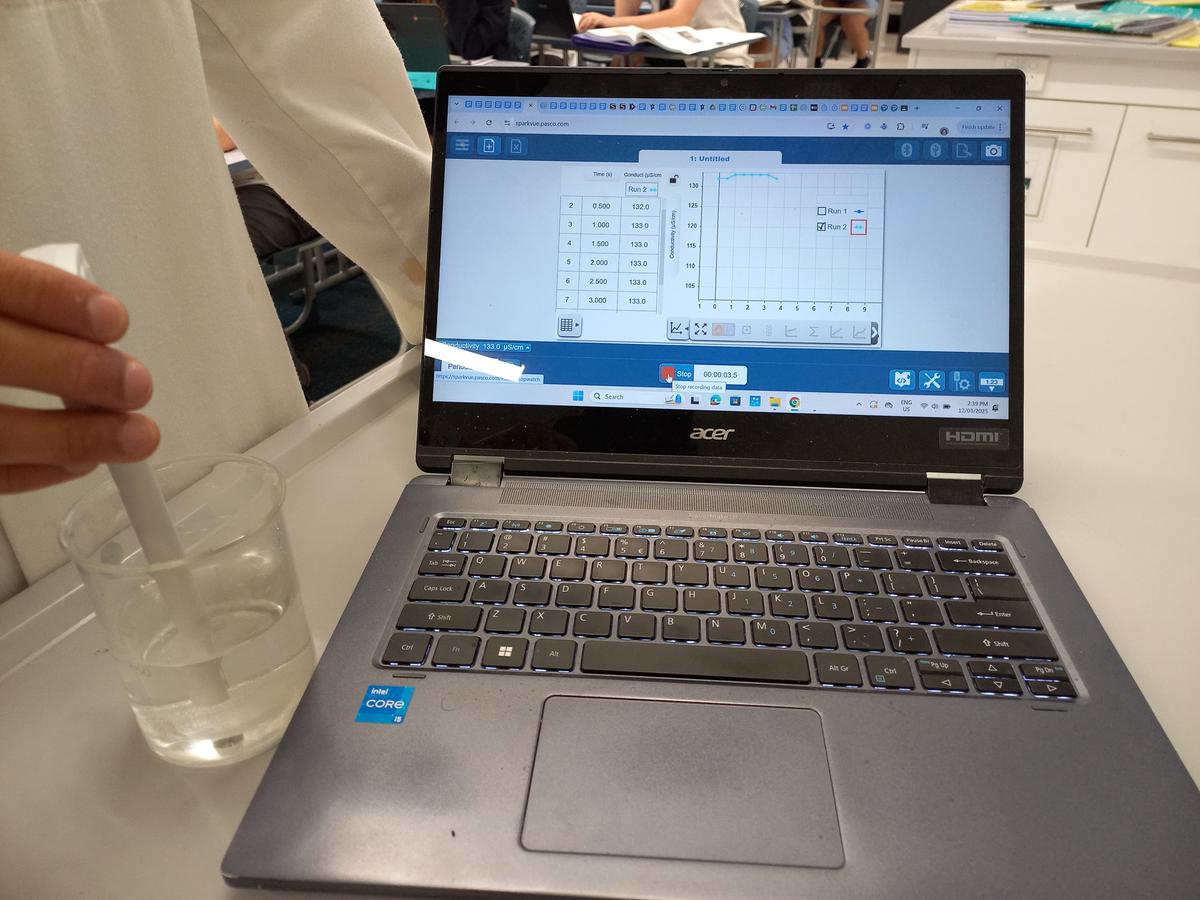
Late last year the Science Faculty purchased a class set of bluetooth conductivity sensors. While electrode technology has been around for decades, only recently has it been possible to purchase a sensor with bluetooth connectivity that connects seamlessly to chromebooks.
This new wireless conductivity sensor has an improved range and decreased error when measuring the electrical conductivity of aqueous solutions. It is ideal for investigating the ionic properties of solutions, including calculations for total dissolved solids (TDS) often used for water quality inquiry. (Further experiments will be developed to tap into this feature.) The new sensors support a doubling of previous conductivity determination, now up to 40,000 µS/cm.
For the students, this is an opportunity to become more familiar with the instruments they will use during their tertiary studies and in their future workplaces.
In a recent experiment, the Year 11 Chemistry students used the sensors to quantitatively differentiate between the conductivity of salt, sugar and paraffin wax as part of a larger number of experiments that examined the relationships between the properties of various materials and their intermolecular bonding.
Thérèse Sweeney
Chemistry teacher
During a recent experiment, our group tested to see the conductivity of sugar, salt and paraffin wax. By heating the substances, we were able to determine the intermolecular bonding holding each substance together based on how quickly they melt. Using a conductivity probe, each solution was tested to determine its conductivity and was put together in a graph. This experiment was super fun as we got to use a new type of technology that had become a lot more accessible for science studies.
Senuly Silva, Year 11
Recently our class did an experiment displaying how the different types of intermolecular bonding changes the physical properties of different substances, namely salt, wax and sugar. Through the experiment I learnt the difference between qualitative and relative data, and the importance of both. The probe used to measure conductivity was able to measure the conductivity with surprising accuracy.
Liam Marryatt, Year 11
During a recent chemistry class, we were fortunate enough to be able to utilise the new technology of a conductivity probe. The probes were fascinating to use and allowed us to understand the different properties of certain common compounds: salt, sugar and wax. We collected data and used it to make judgements regarding their levels of conductivity.
Harley Karro & Chloe Guidera, Year 11
Recently the Year 11 VCE students took part in an exciting experiment, determining key qualities of various substances such as wax, sugar and salt. We began by testing the strength of intermolecular bonds through determining the melting points of the substance. We saw that wax quickly melted highlighting the weak dispersion forces holding it together.
We then used new fascinating equipment; conductivity probes which measured the conductivity of these substances in solutions. The results showed salt to be an amazing conductor in water, the charged anions and cations allowing electricity to flow through the solution.
Ben Knox, Year 11
During a recent class experiment, we examined intermolecular bonding in three different substances: salt, sugar, and paraffin. To compare the differences between these chemicals, we used devices known as conductivity probes. They were highly fascinating because of their advanced technology and precise readings.
Angelina Kaminsky, Year 11
In a recent experiment, we tested the conductivity of various materials using our new probe, which provided us with remarkably accurate information. The precision of our findings has opened up exciting new possibilities for further research and exploration in chemistry.
Adam Salfas, Year 11
Through this experiment, I learned how different types of chemical bonds affect the physical properties of substances. The way salt dissolved and conducted electricity contrasted with sugar and wax, making it clear how ionic and covalent bonds behave differently. I realised the importance of precise experimental methods, especially in measuring conductivity. Small mistakes, such as contamination of the probe, could lead to inaccurate results, highlighting the significance of careful lab procedures.
I also learned how to properly use a conductivity probe to measure the electrical conductivity of solutions. Understanding how to clean the probe between tests and interpret the readings helped me appreciate the accuracy needed in scientific experiments.
Lisa He, Year 11
During a recent experiment, our group used a probe to test the conductivity of salt, sugar and wax. The result shows that dissolved salt has high conductivity due to ion movement.
Elise Liang, Year 11