SCIENCE

YOU DON’T PUT OUT A FIRE WITH OIL
Common sense tells us that liquid water is a much better substance to put out a fire than vegetable oil. But why? Contrary to popular belief, vegetable oil is not actually classified as a flammable liquid and its boiling point is about 300℃(compared to only 100℃for water)! However, the physics of heating and cooling isn’t just about temperature.
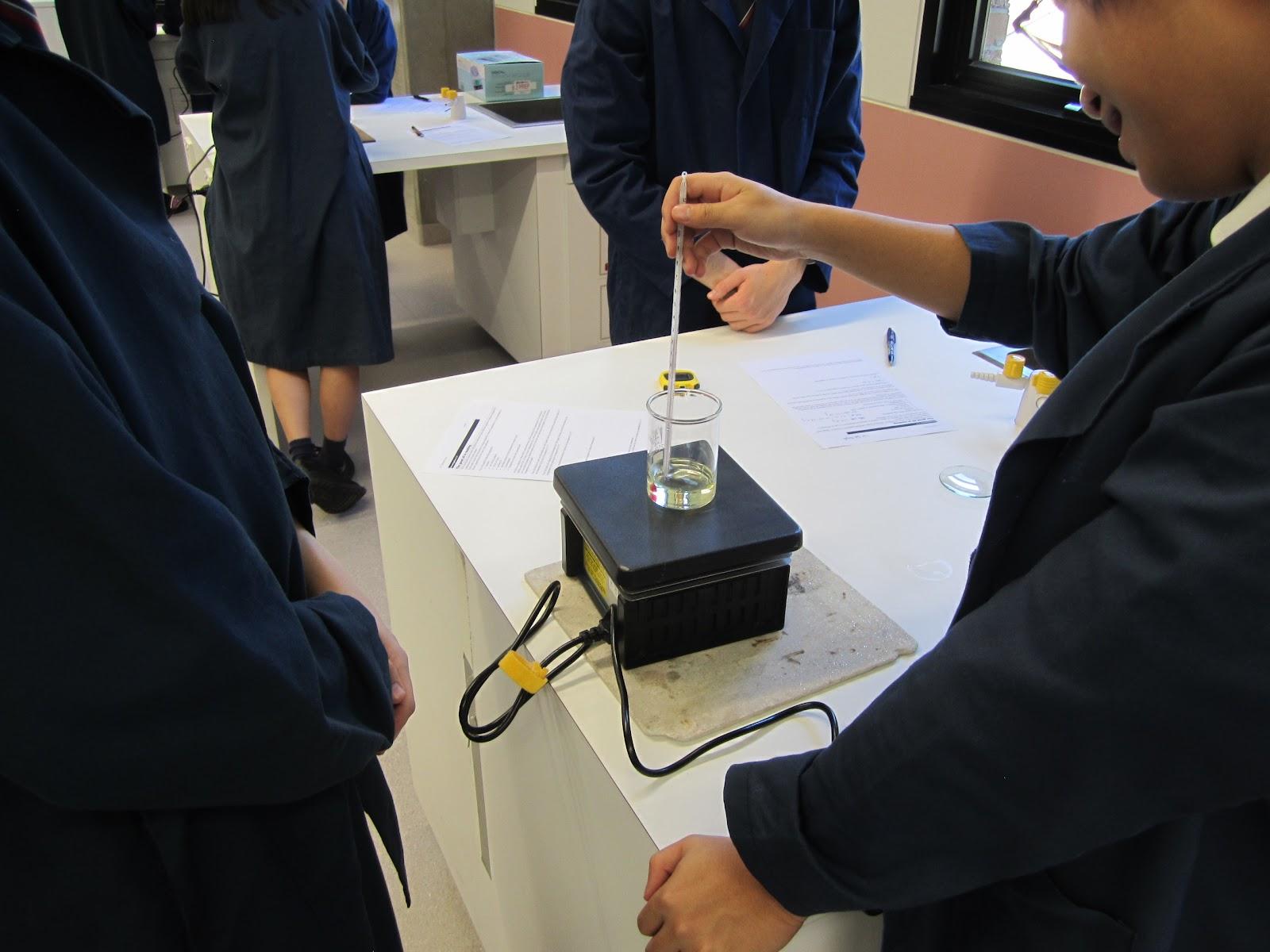
Recently, in the Thermal Physics unit in Year 11, students investigated how much heat is required to raise the temperature of some common liquids. It was found that vegetable oil, despite a slow start (due to its viscosity or how “thick” it is), changed its temperature at a faster rate than water under the constant application of heat.
This observation means that water has the ability to absorb more heat than oil per unit mass. In fact, based on the theory, water should take on 2.5 times more heat energy than oil. Furthermore, by carefully applying heat to different (but known) masses of water and oil, students were able to demonstrate that the specific heat capacity of water is much larger than oil.
As a result, the temperature of the oil will go up much quicker than water if it is heated. That’s why it is hard to put out a fire using oil. Water on the other hand can absorb a lot of heat before its own temperature goes up, and hence, it can rapidly reduce the temperature of a burning object.
Dr S Law
VCE Physics Teacher
Students’ reflection after the activity:
The thermodynamics experiment we did was incredibly enjoyable and entertaining. Our goal was to determine which liquid (water or oil) would heat up by ten degrees. The results were fascinating, and I would recommend that all students in grades 10 and below take physics in Years 11 and 12.
Sai (Year 11G)
Thermal physics is good, you can learn things. Oil will heat up faster than water.
Andreas (Year 11M)
Thermal physics was an easy interactive unit in the 1 & 2 Physics course, and it was fascinating to learn about temperature and heat. My third eye opened upon learning that heat and temperature are very different. We learned about the differences in thermal properties of oil and water, and that their different boiling points are due to their differences in vapour pressure.
Casper (Year 11D)
The outcome of the Heating Prac was as predicted making it really satisfying and the process of this experiment was amusing and entertaining. It allowed us to witness the theory in action. Thermodynamics as a unit was a great study unit and it “heated me up”.
Aryan & Joshua (Year 11F)