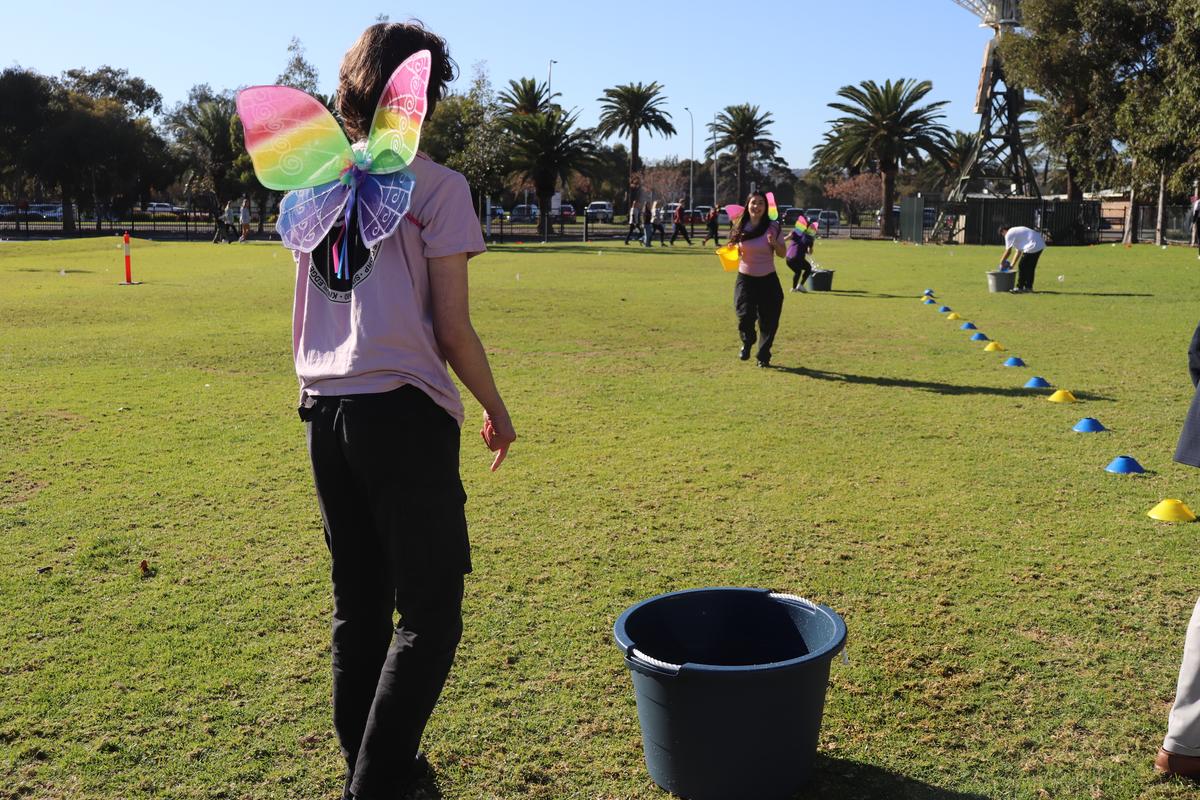Around the College

Relay for Change
We are thrilled to share the incredible success of our Year 12 students in the Relay for Change event, which marked the culmination of their MAX program. Throughout the semester, students have studied four key topics:
exploring their own identity, relationships, community engagement, and their place in the world beyond school.
In Term 2, students embarked on a journey to understand the Christian belief that we are created to live in a harmonious relationship with God and with one another.
12 Therefore, as God’s chosen people, holy and dearly loved, clothe yourselves with compassion, kindness, humility, gentleness, and patience…. 14 And over all these virtues put on love, which binds them all together in perfect unity. (Colossians 3:12-14)
Through the unit 'Giving to Communities' and the Relay for Change event, students have gained a profound understanding of the importance of service, justice, and empathy towards others.
We extend our heartfelt gratitude to all the families, staff, Celia and Josie (ALWS), and community members who supported our
Year 12 students in the Relay for Change event. Together, we raised over $5000, which is a remarkable achievement. This incredible effort will provide over 200 children in East Africa with opportunities to go to school.
A huge well done to all Year 12 students for their active participation and genuine display of love for others. Their unwavering energy and enthusiasm made the Relay for Change event an amazing day.
Special congratulations to Miss Dutton's MAX class, who were the overall winners of the event.
MAX Team
Saponification
Soap making has remained unchanged over the centuries. The ancient Roman tradition called for mixing rainwater, potash and animal tallow (rendered form of beef or mutton fat).
Making soap was a long and arduous process. First, the fat had to be rendered (melted and filtered). Then, potash solution was added. Since water and oil do not mix, this mixture had to be continuously stirred and heated sufficiently to keep the fat melted. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap.
Students enjoyed this practical experiment, and used a lot of creativity when displaying their final product.
Yvonne Hearn
Senior Chemistry Teacher